Jacob Stephens & Benjamin Tartter
Introduction
A lab on chromatography is important to AP Chemistry because chromatography illustrates how compounds can be separated and gives practice in theoretical consideration of polar interactions. This lab provides 5 solvents & 1 compound mixture to students, from which they can create a procedure involving chromatographing with at least two solvents.
The lab we modified can be found in the College Board’s AP 2014 Chemistry Lab Manual, AP Chemistry Guided Inquiry Experiments: Applying the Science Practices. The lab is labeled as “Investigation 5 – Sticky Question: How Do You Separate molecules That Are Attracted to One Another?”
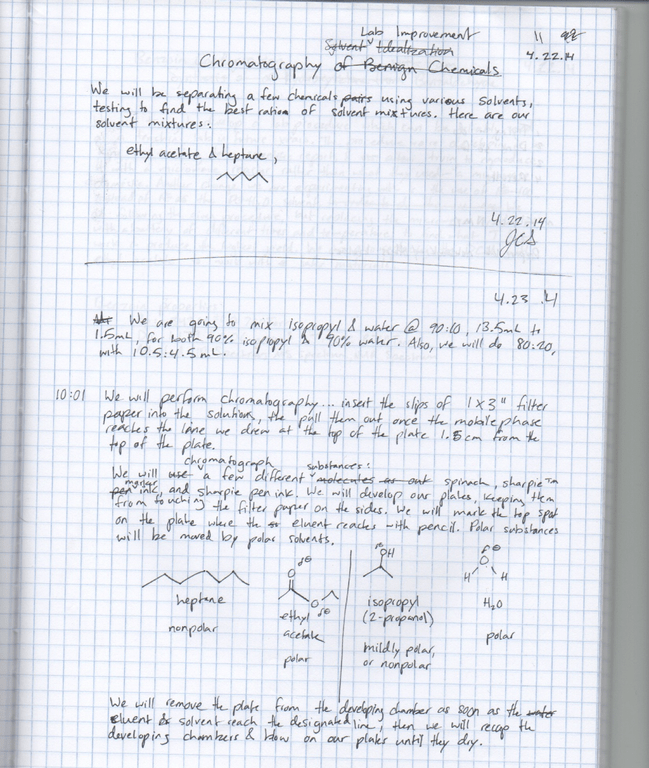
We replaced the hexane/petroleum ether with heptane/ethyl acetate, because these chemicals are safer to human health & the environment. We’re also adding a 6th solvent: isopropanol/H2O. The professor prepares these solvent mixtures before class, & we did research to find the most effective mixture ratios between heptane & ethyl acetate, as well as another solvent we are adding (isopropanol/H2O). The most effective ratio is the one which shows the best separation. Finally, we tested additional compounds to be chromatographed: spinach & marker ink. The reason for this is that the food dyes of the provided lab do not easily breakdown in the environment.
Balanced chemical reactions will be outlined in the discussion of our research.
Materials
- 10 mason jars used as development chambers
- pencil
- metric ruler
- labels
- lab filter paper to make 40 plates (we cut 25 x 75 mm plates from ~6×6” round sheets)
- aluminum foil
- black sharpie marker
- spinach
- 10 ml ethanol
- 37.5 ml heptane
- 37.5 ml ethyl acetate
- 37.5 ml isopropanol
- 37.5 ml H2O
Methods
- Create 10 development chambers; prepare 15 ml mixtures of 90:10, 80:20, & 50:50 heptane/ethyl acetate & H2O/isopropanol, where there are mixtures including the majority chemical of both chemicals included in the mixture (there will be a 90% heptane chamber, as well as a 10% heptane chamber).
- Prepare spinach/ethanol mixture for spotting the spinach. Crush 2 spinach leaves into a beaker with solvent that evaporates quickly (we used ethanol).
- Using glass capillary tubes from a digimelt device, spot the plates. Touch the tube to the surface of the spinach solution, then briefly touch the plate, making sure the spot location is above the line which the plate will be drenched in when inserted into the chamber.
- Allow evaporative solvent to dry off of the plate.
- Line sides of chambers with filter paper, making sure the height of the filter paper is great enough to keep the plates from touching them when inserted into the chamber. We cut 2 inch filter paper linings.
- Seal container with aluminum foil. Allow to sit for 2 hours before chromatographing. This allows the solvent to evenly evaporate throughout the chamber.
- Place plate into the solvent, then watch until solvent reaches a line approximately 1 cm from the top of the plate.
- Remove plate and allow solvent to evaporate off.
- Measure distance from original spot to each distinct moved spot as well as the distances between these spots.
Expected Outcome
Our expected outcome is that the compounds we separated will be just as effectively separated by our solvents. We predict that 50 % heptane/ethyl acetate will yiled the best separation for spinach & ink.
Results
We created two series of development chambers. One set contained ethyl acetate and heptane mix and the other contained water and isopropanol. Each series contained five chambers which differed in solution ratios (9:1, 8:2, 5:5, 2:8, 1:9).
Annotated Bibliography
AP Chemistry Guided Inquiry Experiments: Applying the Science Practices. CollegeBoard. 2013. Print. We used an article from this manual, investigation 5, as the lab which we worked to improve.
Levy, Irv. Conversation. 2014, Wenham.
Cannon, Amy. Interviewed by AP Lab Cohort 2014. 2014, Wenham.
Critiques
- The filter paper strips needed to be cut by hand. They also took a decent amount of the lab time that could have been used on performing experiments. The amount of strips needed ended up being quite a considerable amount.
- The dotting of the paper is a delicate task. Multiple batches of chromatography batches were messed up due to either putting too much material on the paper, or too little.
- The material being analyzed by the chromatography should not be a material that has unknown qualities when the solvents and ratios of them are still being tested. Using three distinct primary colors of food dye mixed together as the material gave us a standard by which we knew when they were fully separated. The colors were also easier to distinguish.
- We should have spent more time analyzing the solvents we used to ensure that they matched the solvents that are currently used for Chromatography. This would have saved us a lot of initial time; also, looking into the ratio that is used in a normal chromatography solvent mixture would have saved time and materials.
- When using heptane over hexane, due to hexane being on a danger scale higher than heptane, the user should still understand that heptane is still hazardous and be careful about inhaling the fumes or working outside of the hood.
- Research team did not give themselves a big enough time window to complete experiments and find a successful replacement for the widely used chromatography solvent mixture.
- The slides came out either streaked, blank or with the entire material moved to the top of the slide with no separation.
Future work
- Strips can be cut outside of the lab and in free time. If a team plans to cut their own strips they should be generous with the amount of strips they predict they will need and cut them outside of the lab at their own leisure.
- Future teams should ensure that they know which solvents would theoretically work best and understand why. They should also have an idea of what ratio should work best theoretically.
- When creating slides, they should practice first by performing an actual chromatography with the usual solvent mixture, so that they can master how the procedure should work. They should be familiar with how the dots look separated from each other, when to pull the plates, how to measure the Rf values, and just how much material should be dotted onto the plate for the best separation and results. This will greatly assist with the corrections being made to the experiment procedure and save a lot of initial time by narrowing down which problems need to be focused on.
- Teams should label both the containment chambers and label the plates with which solvent mixture was used to develop it. Labeling the plates allows the team to come back to them later and analyze which could be further developed into.
- Team should spend first visit to lab ensuring that everything is in place and ready to go; solvents are on hand, containment chambers are clean and labeled, materials to be chromatographed are accounted for, plates are cut and that the tools needed are available. This will set them up to fly through experiments on their next visit.