Dr. Keller & Dr. Topp
BIO260: Proposal
How does diet change microbe diversity in the mammalian gut? Colonies of microbes live on and inside of organisms. Collectively these are referred to as microbiota (Ricou et al. n.d.). Mammalian guts contain communities of microbes. Different nutritional inputs yield varying gut environments, which result in different ecosystems of microbial organisms. In addition to variation in microbiota between individual organisms, the amount of food absorbed by individuals’ digestive systems varies between individuals. How does this vary between a genetically obese and regular mouse? There are a variety of diets and ways to eat. The “average American diet” consists of regular meat, dairy, plant products, and gluten (Smith et al. 2012). Popular diet alterations include removing gluten from one’s diet (gluten-free), and removing animal products, which leaves a diet wholly comprised of plant foods.
Our bodies break down food in the process of digestion. Different types of food are broken down with different levels of ease and difficulty (Milton 1999) (Bureau et al 1999). Eventually digestive system breaks down food into small enough bits that it can pass through intestinal epithelial cells into the bloodstream. Finally the food reaches a point where it is absorbed through intestinal walls and into the blood stream (Wilson 1962). Different input of food yields different output bodily results. For example, an increase in iron intake increases the absorption percent of iron intake (Hettiarachchi et al 2010). In a diet with .59g iron daily, the volunteers absorbed 73% of that iron on average. In the same diet, except for the intake of .20g iron, the volunteers’ bodies absorbed 60% of the Fe consumed. A variety of factors affect intestinal absorption.
A vegan diet results in certain species of bacteria thriving, while others may entirely die out. There are compounds present in meat that are not readily available in the vegan diet, for example, vitamin B12 is often deficient in those with vegan diets (Li 2013) (Zanovec et al 2010). Various bacteria in the colon microbiome produce different compounds important to digestion. For example, in mice, Bifidobacterium breve increase the production of an antimicrobial peptide that helps maintain gut homeostasis (Natividad 2013). The mammal gut is an environment with factors that select for different bacteria. Diet largely affects the condition of the gut environment (Moschen et al 2012).
How do different colonic microbes affect body function? I’m interested in the function of the microorganisms that die out as a result of a gluten-free or vegan diet. I’m also curious if any microorganisms are only present in the gluten-free or vegan diet. I’m looking for knowledge to inform people’s diet choice, particularly if a certain diet has a lack of a beneficial microbe or lack of a pathogenic microbe. I’m curious about how much variation exists between individuals concerning the amount of food absorbed by their digestive system. How does the percentage of absorption change? Differences in bacterial abundance will be measured with PCR.
Each bacterium has a locus on their DNA that codes for 16S ribosomal RNA (rRNA). The sequences are different in these, and that is how I will identify different types of bacteria. The number of 16S rRNA genes that are amplified for each of these species will be used to determine what percentage of the genes amplified are from each of these species (Hong et al. 2008). The steps of polymerase chain reaction (PCR) are as follows, first a primer that correlates with the section of DNA that is being replicated must be the section of DNA that will be replicated must 16S rRNA is part of the structure of the S30 ribosome (Woese 1977). I will need a 16S rRNA template for both bacterial strains that I am measuring. I will perform PCR using DNA from fecal matter of each mouse.
What are the differences between the diets? The omnivorous diet has the greatest nutrient diversity. A key difference between vegan and omnivorous diets is how abundant certain macromolecules are. Compared with an omnivorous diet, a vegan diet contains less fat, and more fiber is present. This affects the body my moving materials through it much more quickly. The concerns associated with vegan diet are primarily that there will be a lack of necessary nutrients. Gluten is primarily a protein present in wheat (Caminero 2012). People who have an autoimmune reaction to gluten are labeled as having celiac disease.
My hypothesis is that diet and obesity will change the abundance of Bacteroides thetaiotaomicron and Peptostreptococcus magnus present in the colon microbiota. Diet will change absorption of food in mice. The vegan diet will result in the least amount of absorption because it contains a large amount of fiber, which M. musculus cannot digest. The obese mouse will absorb a higher percentage of food on diet when compared with the lean mouse. I expect to find a relationship between the abundance of B. thetaiotaomicron and P. magnus and the amount of intestinal absorption.
Methods
To test my
hypothesis that diet and obesity change the composition of the gut microbiota,
I will compare a few different groups of test subjects. My conditions will be obese
and lean mice on vegan, omnivorous, and gluten-free diets. The variable outputs
are the abundances of bacteria and amount of food absorbed. Time is the
variable input. I will use polymerase chain reaction (PCR) for
16S ribosomal RNA to measure the abundance of bacteria, and I will weigh the mice’s
fecal material to measure food absorption.
I will use New
Zealand obese mice (NZO) and New Zealand
black mice (NZB) (Johnson and Hirsch 2013) (Plum et al. 2000). These are
related strains, the major difference: NZO is obese and NZB is lean. One male
and female mouse of each strain will be purchased from jaxmice.jax.org. The two initial
NZO and two initial NZB mice will be bred until there are 30 sexually mature
obese and lean mice (Bruce 1947). The mice will be maintained at 20°C (Noriyuki et al. 2013)
and exposed to 14 hours of fluorescent
light daily. Each mouse will have its own 1.9 L cage with aspen pulp bedding.
The bedding will be changed in each cage every two weeks. The cages will not be
dirty to the point of harming the mice after two weeks (Rosenbaum 2009).
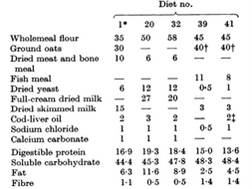
I will have three different diets. The omnivorous diet will be have 40% wholemeal flour, 40% oats, 8% fish meal, 1% dried yeast, 3% dried skim milk, 2% cod liver oil, & 1% sodium chloride (Bruce and Parkes 1949). The second diet will be composed of only plant foods that define the vegan diet. It will be the same as the omnivorous diet, except the 8% of that diet which is fish meal, and the 3% of that diet which is dried skimmed milk will be removed and replaced with 5.5% kidney bean and 5.5% soy bean. These beans do not harm mice (Bradfield et al. 1985). Although yeast is not a plant food, it will be kept in the vegan diet because ordinary vegan diets include bread made with yeast. In addition, I will replace the cod-liver oil with flax seed oil. Next, the gluten free will be the same as the omnivourous diet, with the exception that the wholemeal flour will be replaced with oats, because oats are gluten-free (Thompson 2003). Once per day each food dish will have eight grams of food added.
Table 1. The diet composition I will use is 41 on this graph
Once per week, for 12 weeks, the feces of each mouse will be collected in order to identify the abundance of B. bacteroides and P. magnus. in each conditional group. I will use 16S rRNA PCR to do this. First. I must isolate the bacterial DNA from the stool. I will prepare the PCR templates for B. bacteroides and P. magnus using the methods described by Falsafi et al. (2009) Each week I will do 60 preps.
I’m going to measure a few different types of bacteria. I’ll measure for Bacteroides thetaiotaomicron. These are a beneficial species in the intestines (Canny 2008). The other species I will measure is a gram-positive anaerobic cocci which is common in the small intestine: Peptostreptococcus magnus. I will do multiple PCRs, each will specifically target the individual bacteria of interest.
I will measure food absorption. Daily, I will make sure that there are 15 grams of food in each cage (Louisiana Medical Veterinary Association) at the beginning of the day. Before doing so I will measure how much food is in the cage, and write down the difference between 15 and the amount present in the morning. This way I will know how much food the mice ate the twenty four hours prior to measurement.
This also will require that I daily gather the droppings each mouse leaves and weigh them. By comparing the weight of the stools and the weight eaten from the same day, I will determine how much food was absorbed by each.
In my experiments, I am measuring the difference between the means of how much bacteria exist in each type of mouse for each diet. I am also measuring the means between how much food each type of mouse absorbs intestinally. I have more than two samples, so if my results are parametric I will analyze the results using ANOVA. If my results are non-parametric I will uses the Kruskal-Wallis test. My alpha level will be set at 0.05 for all analyses.
Anticipated
Results
My hypothesis is that vegan and gluten-free diets will change the abundance and presence of various bacterial species present in the colon microbiota. Diet will also change absorption of food in mice. The vegan diet will result in the least amount of absorption because it contains a large amount of fiber, which M. musculus cannot digest. The heavy mouse will absorb a higher percentage of each diet they try when compared with the light mouse.
Here’s the big picture of what I expect, the amount of food absorbed and the change in bacterial abundance depend upon the diet. Obesity should only increase these factors. Bacterial abundance of both B. thetaiotaomicron and P. magnus should increase in obese mice, and more food should be absorbed by those mice.
The obese mice should absorb more what than the lean mice. In 1995, Ferraris and Vinnakota observed that a C57BL/6J ob/ob obese mouse absorbed approximately 100 nmol * cm-1 * min-1 more of glucose and proline than its lean counterpart. Likewise, the NZO mice should absorb more food than their lean counterparts for each diet. This will be determined by comparing the food weight consumed with the stool weight. For lean and obese, I expect the vegan diet to have the highest percent total food weight absorbed. This is because vegetables contain more fiber than animal products.
Table 1. Expected daily absorption for each condition. The obese mouse should absorb 50% more food than the lean mouse for each diet (Ferraris and Vinnakota 1995). 1.5x=y. x=lean, y=obese. The gluten-free diet should have the most digestion because the lack of gluten make for a higher percentage of absorbable nutrients. Although the mouse can break down gluten, all of it will probably not break down. The vegan diet should have the least absorption because it has the most indigestible fiber.
|
|
Vegan (g/day) |
Omnivore (g/day) |
Gluten-free (g/day) |
|
Lean |
3.3 |
4 |
4.7 |
|
Obese |
5 |
6 |
7 |
The plant diet should have the highest abundance of Bacteroides thetaiotaomicron in both mice. B. thetaiotaomicron metabolizes plant polysaccharides (Mirza 2011). The vegan diet has a higher percentage of plant food, meaning more plant polysaccharides for the B. thetaiotaomicron to eat, resulting in greater rates of growth. Obese mice have greater intestinal absorptive capacity than lean mice because of greater intestinal surface area (Ferraris and Vinnakota 1995). Greater instestinal wall area creates a higher rate of absorption. They do not differ in the absorption per mg of intestinal tissue. Obese mice should have a greater abundance of B. thetaiotaomicron, because the area for them to colonize will be increased. Also, a different obese strain of mouse, C57BL/6J ob/ob, eats 64% more on average than it’s lean counterpart (Ferraris and Vinnakota 1995). Likewise, the NZO mouse should eat more than the NZB, and thus provides a greater food supply for B. thetaiotaomicron, resulting in increased abundance.
The gluten free diet will increase the abundance of B. thetaiotaomicron if oats contain more polysaccharides than wheat. The obese mouse ought to absorb more material from the gluten free diet (GFD). Gluten is difficult to digest, so it must be hard for animals’ digestive tracts to break it down entirely. Some gluten must be left over and then pass through the system. Compare this to the GF diet, which does not have gluten proteins to break down. The mice should digest the highest percent of the GF diet’s food weight.
(a) (b)
Figure 1. Abundance of Bacteroides thetaiotaomicron in lean (a) and obese (b) mice on gluten-free, omnivorous, and vegan diets.
I expect the diet with the most protein to result in the highest abundance of gram-positive anaerobic cocci (GPAC). The diet with the highest protein will be the omnivorous diet. Greater protein should result in more GPAC because they feed on products of protein degradation (Murdoch 1998). In the omnivorous diet the most products of protein degradation (peptides and amino acids) will be available for the GPAC to metabolize (Silk 1974).
I expect the obese mouse to have the more GPAC than the lean mouse. Due to greater intestinal area, I expect the amount of digestive enzymes secreted by the pancreas to be higher. As a result of increased digestive enzymes and a greater quantity of food, I expect there to be more amino acids. The greater intestinal area in the obese mouse means there should be a greater amount of absorption, which will decrease the amount of amino acids available for the GPAC. However, because they are outside of the mucosal lining of the intestines, they will be able to get at the food before it is absorbed. The GPAC will be more abundant in the obese mouse than the lean mouse.
How long do I expect the gut genome to change?
(a) GPAC in lean mice (b) GPAC in obese mice
Figure 1. Abundance of gram-positive anaerobic cocci in lean (a) and obese (b) mice on gluten-free, omnivorous, and vegan diets.
Thus we come to the end of the experiment. Hopefully I will find with this study that all bacteria I’m measuring are in greater abundance in obese mice than lean mice. B. thetaiotaomicron should be found in the greatest abundance in the vegan diet, and gram-positive anaerobic cocci should be in the greatest abudance for the omnivorous diet. Concerning intestinal absorption. This study will make progess in determing the relationship between absorption and abundance of B. thetaiotaomicron and P. magnus. Future work could include research into the relationship between specific nutrients and these bacteria, or the relationship between diet and more pathogenic bacteria. That could have implications for evidence based medicine, in addition to prescribing an antibiotic, physicians could also prescribe a certain diet that would decrease the abundance of a pathogenic bacteria.
Cost
Each mouse will cost $125 each from jaxmice.org. I need one male and female mouse of each breed, so the total cost for mice will be $500.
100kg of food for the mice, regular mouse feed for the 20 days of four mice (1.2kg food), 25 days of 24 mice (9kg), 25 days of 44 mice (16.5kg), 50 days of 64 mice (48kg). For this I need 74.7kg of mouse feed, which equates to 164lbs of mouse food. From myparrotfood.com 40lb bags of mouse feed are $65. I will need 4 bags of this and a four pound bag for $10.73. Shipping is free, so this will cost $270.73.
For the 70 days of the experiment I will use the diets requiring wholewheat flour, oats, beans, cod liver oil, fish meal, dried yeast, salt, and milk powder for sixty mice. 20 will be on the vegan diet, 20 on the omnivorous diet, and 20 on the gluten-free, aka, oat diet. I estimate need 21kg of food per condition at 15g of food per day. 16.8kg of oats for the omnivorous diet, and 16.8kg for the other two. To buy 33.6kg of oats (73lb) from pleasanthillgrain.com I will purchase two $110.75 thirty six pound pails of oat groats. I will need 16.8kg of wholemeal flour (36.5lb). From kingarthurflour.com a 25lb bag of flour costs $21.50, a 10lb bag costs $8.95, and a 5lb bag costs $4.95, which totals to $35.40. 3.36kg of fish meal (7.4lb) can be acquired via a 10lb bag of fish meal from amazon for $20.46 + $12.49 shipping, this results in $32.94. I estimate the dried skim milk, yeast, sodium chloride, and cod liver oil to cost $40. Food will cost $329.84 for the experimental groups.
Sixty 1.9L cages will cost $475.80 from wayfair.com.
Gordon college will provide the PCR and associated microbiology components.
Total cost will amount to $1576.37.
Timeline
Breeding the mice will take months. Estrous cycles are 2-5 days on average, with a 12 hour window of fertility in that time span (Louisiana Medical Veterinary Association). Gestation takes 20 days on average, and I am going to keep the male mice with the females during their fertile postpartum estrus 12-24 hours after they give birth in hopes of their becoming pregnant. Concurrent pregnancy and lactation leads to longer gestation time, and does not harm the mice (Johnson et al. 2001). Average litter size is ten to twelve. If I start with a male and female NZB and NZO mouse, the first generation should be 10 mice of each breed, then if the parents become pregnant within a day of giving birth, the next pregnancy may take 25 days. At that point, about 50 days should have passed, assuming that pregnancies occur at the first possible times. “Breeding onset is at about 50 days of age in both females and males” (Louisiana Medical Veterinary Association). By 50 days there should be a 25 day old litter of 10 pups, and a day old litter of 10 pups. I need thirty for each group, so the third generation from the original parents should give me enough pups. 75 days after I acquire the initial mice I should have 30 mice. The third generation will take 50 days to sexual maturity, so I will wait until this point to begin the experiment. Ideally, breeding will take 125 days.
Once I have all the mice, I will put them into individual cages and carry out the experiment for ten weeks, 70 days.
Analyzing the data I expect to take a week, but the experiment itself, from the day that all materials are gathered (at least the materials needed to begin the breeding), the experiment should take 195 days.
Bibliography
Anon, A. 2013 "Mouse Husbandry, Breeding
and Development". University of Carolina, Irvine, Transgenic Mouse
Facility Guidelines. University of Carolina. Retrieved 15 December 2013.
Bradfield, C. A., Chang, Y., & Bjeldanes,
L. F. 1985. Effects of commonly consumed vegetables on hepatic
xenobiotic-metabolizing enzymes in the mouse. Food and Chemical Toxicology 23:
899-904.
Bruce, H. M. 1947. The feeding and breeding of laboratory animals: VI. The breeding of mice. The Journal of Hygiene 45: 420.
Bruce,
H.M., and A.S. Parkes. 1949. Feeding
and breeding of laboratory animals IX. A complete cubed diet for mice
and rats. Journal of Hygiene 47:202-208.
Bruce, H.M., and C.W. Emmens. 1948. The feeding and breeding of laboratory animals VII. Methods of testing the adequacy of diets for breeding mice. Journal of Hygiene 46: 315-324.
Bureau, D. P., A.M. Harris, and C.Y. Cho. 1999. Apparent digestibility of rendered animal protein ingredients for rainbow trout (Oncorhynchus mykiss). Aquaculture 180: 345-358.
Caminero, A., E. Nistal., L. Arias, S. Vivas, I. Comino, A. Real, C. Sousa, J.M. Ruiz de Morales, M.A. Ferrero, and J. Casqueiro. (2012). A gluten metabolism study in healthy individuals shows the presence of faecal glutenasic activity. European Journal Of Nutrition 51: 293-299.
Canny, G. O., & McCormick, B. A. 2008. Bacteria in the intestine, helpful residents or enemies from within?. Infection and immunity 76: 3360-3373.
Claesson, M. J., Jeffery, I. B., Conde, S., Power, S. E., O’Connor, E. M., Cusack, S., & … Stanton, C. 2012. Gut microbiota composition correlates with diet and health in the elderly. Nature 488: 178-184.
Falsafi, T. Favaedi R., Mahjoub F., Najafi M. 2009. Application of stool-PCR test for diagnosis of Helicobacter pylori infection in children. World Journal of Gastroenterology 15:484-488.
Ferraris, R. P., & Vinnakota, R. R. 1995. Intestinal nutrient transport in genetically obese mice. The American journal of clinical nutrition 62: 540-546.
Hettiarachchi, M., C. Liyanage, D. Hilmers, I. Griffin, and S.A. Abrams 2010. Changing the zinc: iron ratio in a cereal-based nutritional supplement has no effect on percent absorption of iron and zinc in Sri Lankan children. British journal of nutrition 103.7: 1015.
Hong, P. Y., Wu, J. H., & Liu, W. T. 2008. Relative abundance of Bacteroides spp. in stools and wastewaters as determined by hierarchical oligonucleotide primer extension. Applied and environmental microbiology 74: 2882-2893.
Johnson, M.S., Thomson, S.C., Speakman, J.R. 2001. Limits to sustained energy intake: III. Effects of concurrent pregnancy and lactation in Mus musculus. Journal of Experimental Biology 204: 1947-1956.
Johnson, P. R., J. Hirsch. 2013. Cellularity
of adipose depots in six strains of genetically obese mice. Journal of Lipid
Science 13: 2-11.
Li,
Duo. 2013 Effect of the vegetarian diet on non‐communicable diseases. Journal of
the Science of Food and Agriculture 10.1002/jsfa.6362.
Louisiana Medical Veterinary Association. 2013. Biology of the Mouse. Available from: (http://www.lvma.org/ForPetOwners/PublicInterest/EducationMaterial/BiologyoftheMouse.aspx). Accessed: (17 December 2013).
Milton, K. 1999. Nutritional characteristics of wild primate foods: do the diets of our closest living relatives have lessons for us?. Nutrition 15.6: 488-498.
Mirza, B. 2011. Bacteroides thetaiotaomicron. Available from: (http://microbewiki.kenyon.edu/index.php/Bacteroides_thetaiotaomicron). Accessed on: 23 Nov. 2013
Molla, A., Molla, A. M., Sarker, S. A., & Khatun, M. 1983. Whole-gut transit time and its relationship to absorption of macronutrients during diarrhoea and after recovery. Scandinavian journal of gastroenterology 18: 537-543.
Moschen, Alexander R., Verena Wieser, and Herbert Tilg. 2012. Dietary factors: major regulators of the gut’s microbiota. Gut and liver 6.4: 411-416.
Murdoch, D. A. 1998. Gram-positive anaerobic cocci. Clinical microbiology reviews 11: 81-120.
Natividad, Jane MM, et al. 2013. Differential induction of antimicrobial REGIII by intestinal microbiota and Bifidobacterium breve NCC2950. Applied and Environmental Microbiology AEM-02470.
Noriyuki,
K., N. Dai, H. Shin-ichi, T. Saya, K. Motoi, H. Norihiko, S. Mitsuyuki, and A
Fumitoshi. 2013. Shortened blood
coagulation times in genetically obese rats and diet-induced obese mice. The
Japanese Society of Veterinary Science 75: 1245-1248.
Plum, L., R. Kluge, K. Giesen, J. Altmuller,
J.R. Ortlepp, and H. Joost. 2000. Type 2 diabetes–like hyperglycemia in
a backcross model of NZO and SJL mice characterization of a susceptibility
locus on chromosome 4 and its relation with obesity. Diabetes
49:1590-1596.
Price, C.T., J.R. Langford, F.A. Liporace. 2012. Essential nutrients for bone health and a review of their availability in the average North American diet. Open Orthopaedics Journal 6:143–149.
Reece, J.B., L.A. Urry, M.L. Cain, S.A. Wasserman, P.V. Minorsky, & R.B. Jackson. 2009. Mutualistic Bacteria. Page 571 in: Campbell Biology, 9th ed. Pearson Benjamin Cummings, San Francisco, CA.
Rosenbaum, M.D., VandeWoude S., Johnson, T.E. 2009. Effects of cage-change frequency and bedding volume on mice and their microenvironment. Journal for American Association of Lab Science 48:763-773.
Ricou J., S.H. Lee, Studio Graphiko. n.d. “Nature Special: Human Microbiota,” Available from (http://www.nature.com/nature/focus/humanmicrobiota/) Accessed: (Oct. 19, 2013)
Shen, L. L., Xiao, M. M., Kong, F. F., Brown, M. M., Sun, J. J., Kong, Q. Q., & … Ni, X. X. (2011). Detection of Laribacter hongkongensis using species-specific duplex PCR assays targeting the 16S rRNA gene and the 16S-23S rRNA intergenic spacer region (ISR). Journal Of Applied Microbiology, 111: 625-630. doi:10.1111/j.1365-2672.2011.05083.x
Silk, D. B. 1974. Progress report. Peptide absorption in man. Gut, 15: 494-501.
Thompson, T. 2003. Oats and the gluten-free diet. Journal of the American Dietetic Association 103: 376-379.
Van Zwet, A.A., J.C. Thijs, A M Kooistra-Smid, J Schirm and J A Snijder. 1994. Use of PCR with feces for detection of helicobacter pylori infections in patients. Journal of Clinical Microbiology 32:1346.
Woese, C. R.; G. E. Fox (1977). "Phylogenetic structure of the prokaryotic domain: The primary kingdoms". Proceedings of the National Academy of Sciences 74: 5088–5090.
Zanovec, Michael, et al. 2010. Lean beef contributes significant amounts of key nutrients to the diets of US adults: National Health and Nutrition Examination Survey 1999-2004. Nutrition Research 30.6: 375-381.